Langerhans cell histiocytosis (LCH) in adult patients is a rare disease with protean manifestations, including single system unifocal (SSU) or multifocal (SSM) presentation or multisystemic (MS) involvement. Clinical care of adult patients is based on case series and reports. Regimens applied in pediatric patients show unacceptable toxicity in the adult setting.
Our single-center experience with LCH is presented. Data on 30 patients diagnosed between 1997 and 2022 have been reviewed. Patients with isolated pulmonary histiocytosis were excluded. Responses to any treatment were classified according to the acknowledged criteria. Progression-free survival (PFS) after frontline treatment was calculated on all treated patients. Overall survival (OS) was determined on the whole cohort irrespective of any treatment received.
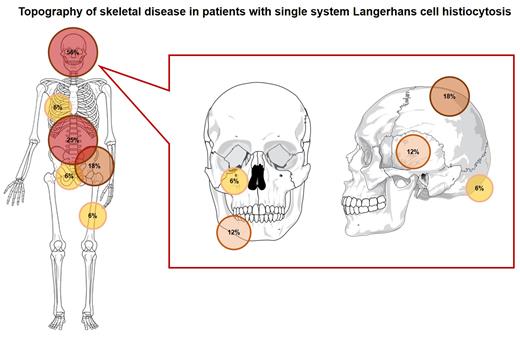
Eighteen male and 12 female patients received a diagnosis of LCH at a median age of 46 years (range 20-75). Sixteen patients had single system (SS) disease, with SSU involvement in 12 cases and SSM involvement in 4 cases. The skeleton was the mostly involved system (Figure), while SSU extra-skeletal disease was detected in 25% of cases. Fourteen patients had MS disease: axial skeleton, lungs, hypophysis, skull, skin and appendicular skeleton were the predominantly involved sites, in this order. Ten patients with SS disease received chemotherapy because of symptoms (mostly bone pain) or due to multifocal dissemination. Two patients underwent radiotherapy (RT), 2 had their disease completely excised and 1 received systemic steroids. One patient was observed. A complete response (CR) was obtained in 60% of treated cases, while a partial response (PR) in 27%. Four patients relapsed after frontline therapy and were rescued with RT or autologous transplant. Among patients with MS disease, chemotherapy (MACOP/VNCOP-B, cladribine, vinblastine) was applied in 6 cases; 2 patients received RT and 1 systemic steroids. One patient underwent surgery and 4 were observed because of asymptomatic disease. A CR was obtained in 70% of treated patients and a PR in 10%. Five patients relapsed, with symptomatic disease requiring treatment (chemotherapy and RT) in 3 cases. Median PFS among all treated patients was 177 months. OS was 91% at 20 years.
Appropriate treatment based on disease extension, site involvement and clinical manifestations yields high remission rates and long term PFS and OS. The optimal approach to relapsed patients remains undefined.
Disclosures
Casadei:Novartis: Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Membership on an entity's Board of Directors or advisory committees; Roche: Speakers Bureau; Lilly: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Zinzani:GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal